Meet the green kleptos of the animal kingdom
Chloroplast-stealing sea slugs straddle the line between plant and animal, holding promise for green energy sources.
Hannah Loss • December 3, 2021
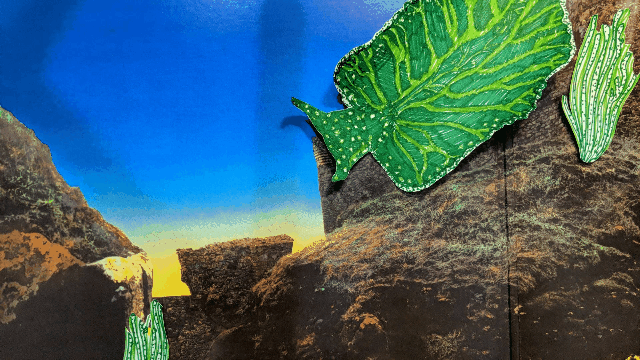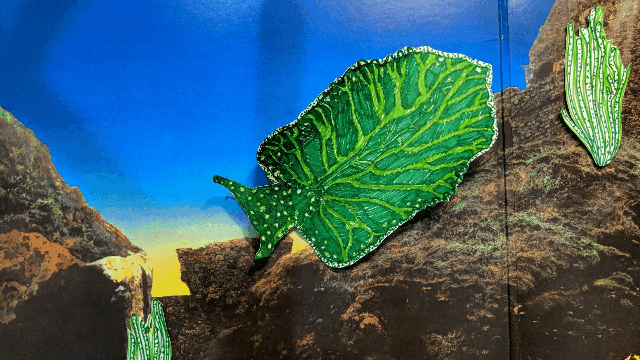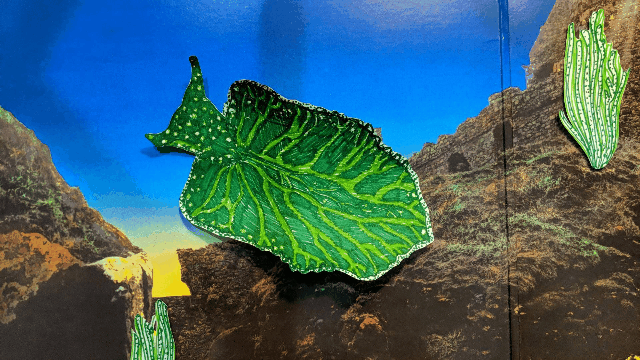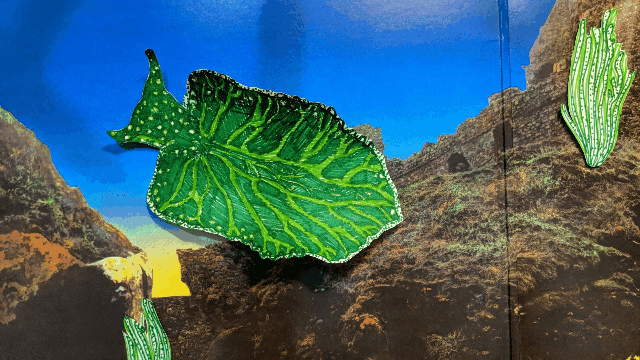
Elysia chlorotica lives in the salt marshes off Martha’s Vineyard and along the U.S. east coast. [Illustration by Hannah Loss]
What if an animal could be more like a plant?
What if cows could skip their grassy meal and perform photosynthesis, absorbing energy by lazing around in the sunlight? What if yeast cells could similarly power themselves without having to be fed, opening up exciting new possibilities for synthetic drug development?
If that all sounds too weird to consider, meet Elysia chlorotica, an animal that resembles a plant.
Also called the eastern emerald elysia, it’s a bright green sea slug about the size of a lemon that lives along the east coast of North America in intertidal marshes, between the land and sea. This boneless, slimy critter has evolved a unique trick that intrigues biology researchers who hope to improve human health and the environment: it steals chloroplasts.
Chloroplasts are the tiny generators of plant cells, where photosynthesis turns sunlight into energy. Some sea slugs, including the emerald elysia, have managed to incorporate chloroplasts into its cells, allowing it to live for months without ingesting any food at all. This process was confirmed in one recently published study in which exposure to sunlight prolonged a sea slug’s life during starvation.
Scientists are still mystified about how the sea slugs manage to photosynthesize after snatching the chloroplasts. But they hope studying them will someday lead to better drugs and fuels, and also generate new insights into how the first algae and plants evolved over a billion years ago.
An emerald elysia absorbs the chloroplasts from algae, after using its tongue covered in little teeth to punch a hole in algae filament and then suck out the contents for food.
“It’s exactly like drinking out of a soda straw,” says Sidney Pierce, a retired biologist at the University of South Florida who has studied sea slugs since the 1970s.
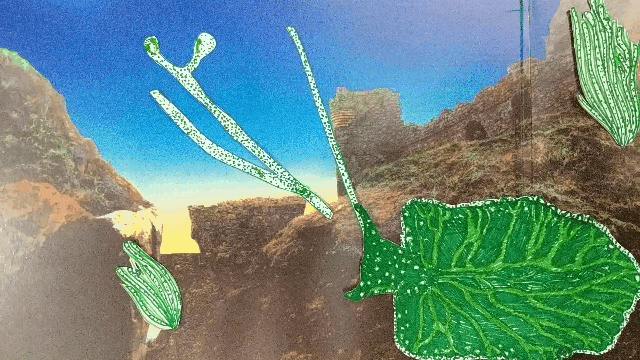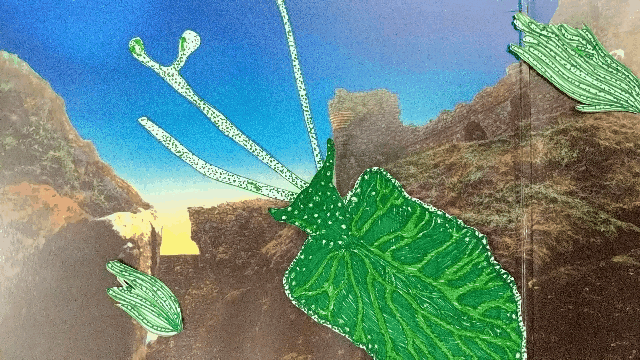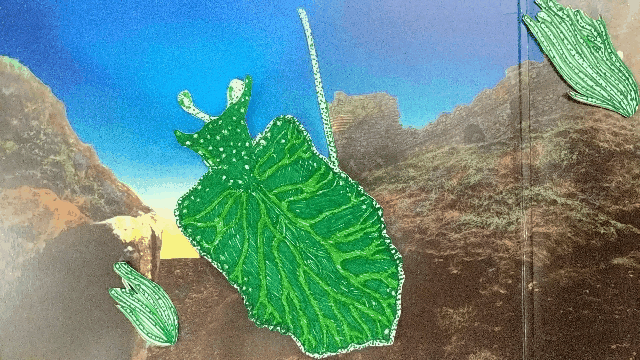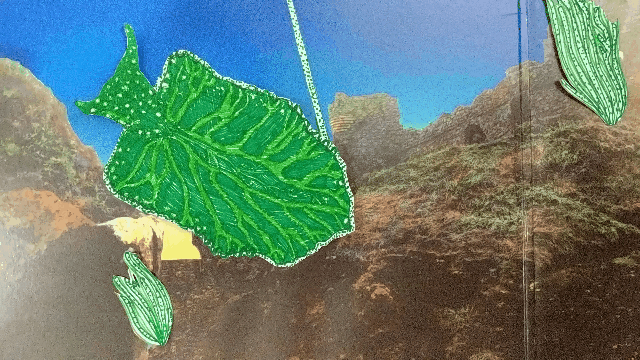
Elysia chlorotica eats algae that grow in long filaments. The sea slug sucks out the algae insides, like drinking from a straw.
The slug digests all of the juicy contents of the algae cells except the chloroplasts, which instead are integrated into the slug’s own cells and somehow continue to photosynthesize effectively, even though they’re living inside a completely foreign animal cell.

A chloroplast enters the sea slugs’ cell membrane and is integrated into the cytoplasm.
How do they do it? “We don’t know at all,” says Pierce, “that’s the interesting part.”
He’s not the only one who’s intrigued. Back when Joshua Widhalm was a graduate student, he was sitting in a genomics and evolution class when his professor challenged the students to define a plant. Easy enough, he thought: anything that has chloroplasts and uses photosynthesis for energy.
But then his professor projected an image of an eastern emerald elysia, its soft body speckled with green dots — chloroplasts. It was an animal that photosynthesizes.
“I had never heard of these sea slugs before, and I thought it was the coolest thing I’d ever seen,” recalled Widhalm, now a cell biologist at Purdue University in West Lafayette, Indiana.
Today, he’s more fascinated with the slugs than ever. So interesting, in fact, that his current research focuses on how a similar species, Elysia clarki, manages to incorporate chloroplasts into their own cells through a process called “kleptoplasty”.
Pierce theorizes that the slugs manage the trick by stealing not only the algae’s chloroplasts, but also some of its genes, which are then incorporated into their own DNA. The theory would explain his 2009 discovery that sea slug cells somehow produce algal proteins. Those proteins are important because algae and plants use them to repair chloroplasts that are damaged by sunlight radiation during photosynthesis. Animal cells typically don’t create those plant-specific proteins — but the sea slugs do, perhaps because they use the plant genes acquired from ingesting algal DNA.
If he’s right, Pierce thinks working out the mechanics of kleptoplasty could have important applications for gene therapy, which involves adding, removing or changing cellular genetic material to treat human diseases.
But Pierce’s gene-transfer idea isn’t the only hypothesis. A second idea is that the chloroplasts remain genetically separate from the slug, but still function because they don’t need the same level of support from their host that other kinds of chloroplasts do.
What especially excites Purdue’s Widhalm is that the sea slug research shows introducing plant-like characteristics into an animal may be possible. “There’s a molecular blueprint” for bringing new components into cells, he says.
Widhalm imagines a far-off possible future where the same mechanism is used to add chlorophyll to livestock so they can get part of their food from the sun, although he says ethical concerns for engineering animals would need to be addressed. A more immediate application might be adding chlorophyll to yeast cells used for creating drug products, so that the yeasts require less fuel and can be grown more efficiently.
Studying kleptoplasty may also help biologists figure out one of the “grand challenges” of their field, says Widhalm: It could be a model of early plant evolution. Roughly over a billion years ago, chloroplasts first integrated into what would become modern plants and algae cells. Before that, photosynthesis only occurred in single-celled bacteria. Researchers speculate that another ancient single-celled organism engulfed these bacteria, and after millions of years of evolution, these bacteria settled into their new host to become the plant chloroplasts we know today.
Understanding how this evolutionary step occurred could bolster knowledge of how to treat human mitochondrial diseases. The defunct part of the cell, or diseased mitochondria, could be replaced by healthy ones through a similar mechanism, says Widhalm.
He and his collaborators at MIT and University of California, Berkeley are developing a strategy to use tiny cylindrical molecules made of carbon atoms to pierce cell walls to make genetic changes to the material inside. Their goal is to “knock out,” or turn off, certain genes inside the slugs and chloroplasts to test which ones might be necessary for slugs to harbor the stolen chloroplasts.
After nearly 30 years of research, understanding the mechanisms of kleptoplasty in sea slugs still excites and eludes Sidney Pierce — so much so that if he were starting over, he’d do the same thing: “If I was a 30-year-old fresh off the press Ph.D., that’s what I’d be working on right now.”
1 Comment
This is fabulously interesting! Your writing makes me want to learn more… Living this style!